Xenon And Krypton Chemical Formula . how noble gases form compounds. Helium, neon, argon, krypton, xenon, radon have completed valence electron shells, so they are highly stable. The elements are helium (he), neon (ne), argon (ar),. Stable compounds of xenon form when xenon. krypton, compound with xenon (1:1) formula: noble gas, any of the seven chemical elements that make up group 18 (viiia) of the periodic table. krypton forms a difluoride, krf 2, which is thermally unstable at room temperature. xenon (xe), chemical element, a heavy and extremely rare gas of group 18 (noble gases) of the periodic table. this article contains comparison of key thermal and atomic properties of krypton and xenon, two comparable chemical elements from the periodic table. It was the first noble gas found to form true chemical.
from utedzz.blogspot.com
how noble gases form compounds. xenon (xe), chemical element, a heavy and extremely rare gas of group 18 (noble gases) of the periodic table. krypton forms a difluoride, krf 2, which is thermally unstable at room temperature. The elements are helium (he), neon (ne), argon (ar),. krypton, compound with xenon (1:1) formula: this article contains comparison of key thermal and atomic properties of krypton and xenon, two comparable chemical elements from the periodic table. noble gas, any of the seven chemical elements that make up group 18 (viiia) of the periodic table. Helium, neon, argon, krypton, xenon, radon have completed valence electron shells, so they are highly stable. Stable compounds of xenon form when xenon. It was the first noble gas found to form true chemical.
Periodic Table Krypton Protons Periodic Table Timeline
Xenon And Krypton Chemical Formula krypton forms a difluoride, krf 2, which is thermally unstable at room temperature. xenon (xe), chemical element, a heavy and extremely rare gas of group 18 (noble gases) of the periodic table. how noble gases form compounds. The elements are helium (he), neon (ne), argon (ar),. noble gas, any of the seven chemical elements that make up group 18 (viiia) of the periodic table. Helium, neon, argon, krypton, xenon, radon have completed valence electron shells, so they are highly stable. Stable compounds of xenon form when xenon. It was the first noble gas found to form true chemical. krypton, compound with xenon (1:1) formula: krypton forms a difluoride, krf 2, which is thermally unstable at room temperature. this article contains comparison of key thermal and atomic properties of krypton and xenon, two comparable chemical elements from the periodic table.
From ar.inspiredpencil.com
Element Krypton Xenon And Krypton Chemical Formula Stable compounds of xenon form when xenon. noble gas, any of the seven chemical elements that make up group 18 (viiia) of the periodic table. The elements are helium (he), neon (ne), argon (ar),. It was the first noble gas found to form true chemical. xenon (xe), chemical element, a heavy and extremely rare gas of group 18. Xenon And Krypton Chemical Formula.
From www.animalia-life.club
Atomic Structure Of Krypton Xenon And Krypton Chemical Formula krypton, compound with xenon (1:1) formula: noble gas, any of the seven chemical elements that make up group 18 (viiia) of the periodic table. It was the first noble gas found to form true chemical. The elements are helium (he), neon (ne), argon (ar),. xenon (xe), chemical element, a heavy and extremely rare gas of group 18. Xenon And Krypton Chemical Formula.
From cartoondealer.com
Krypton Chemical Element Vector Illustration 83098754 Xenon And Krypton Chemical Formula xenon (xe), chemical element, a heavy and extremely rare gas of group 18 (noble gases) of the periodic table. Stable compounds of xenon form when xenon. The elements are helium (he), neon (ne), argon (ar),. krypton forms a difluoride, krf 2, which is thermally unstable at room temperature. It was the first noble gas found to form true. Xenon And Krypton Chemical Formula.
From www.americanelements.com
Xenon (Xe) AMERICAN ELEMENTS Xenon And Krypton Chemical Formula The elements are helium (he), neon (ne), argon (ar),. how noble gases form compounds. Helium, neon, argon, krypton, xenon, radon have completed valence electron shells, so they are highly stable. It was the first noble gas found to form true chemical. Stable compounds of xenon form when xenon. krypton, compound with xenon (1:1) formula: this article contains. Xenon And Krypton Chemical Formula.
From material-properties.org
Krypton and Xenon Comparison Properties Material Properties Xenon And Krypton Chemical Formula noble gas, any of the seven chemical elements that make up group 18 (viiia) of the periodic table. krypton forms a difluoride, krf 2, which is thermally unstable at room temperature. this article contains comparison of key thermal and atomic properties of krypton and xenon, two comparable chemical elements from the periodic table. how noble gases. Xenon And Krypton Chemical Formula.
From ar.inspiredpencil.com
Xenon Electron Dot Structure Xenon And Krypton Chemical Formula noble gas, any of the seven chemical elements that make up group 18 (viiia) of the periodic table. how noble gases form compounds. xenon (xe), chemical element, a heavy and extremely rare gas of group 18 (noble gases) of the periodic table. Stable compounds of xenon form when xenon. krypton, compound with xenon (1:1) formula: It. Xenon And Krypton Chemical Formula.
From material-properties.org
Xenón Tabla periódica y propiedades atómicas Xenon And Krypton Chemical Formula krypton, compound with xenon (1:1) formula: Stable compounds of xenon form when xenon. The elements are helium (he), neon (ne), argon (ar),. this article contains comparison of key thermal and atomic properties of krypton and xenon, two comparable chemical elements from the periodic table. Helium, neon, argon, krypton, xenon, radon have completed valence electron shells, so they are. Xenon And Krypton Chemical Formula.
From utedzz.blogspot.com
Periodic Table Krypton Protons Periodic Table Timeline Xenon And Krypton Chemical Formula xenon (xe), chemical element, a heavy and extremely rare gas of group 18 (noble gases) of the periodic table. noble gas, any of the seven chemical elements that make up group 18 (viiia) of the periodic table. this article contains comparison of key thermal and atomic properties of krypton and xenon, two comparable chemical elements from the. Xenon And Krypton Chemical Formula.
From www.schoolmykids.com
Xenon (Xe) Element Information, Facts, Properties, Uses Periodic Table of the Elements Xenon And Krypton Chemical Formula xenon (xe), chemical element, a heavy and extremely rare gas of group 18 (noble gases) of the periodic table. Helium, neon, argon, krypton, xenon, radon have completed valence electron shells, so they are highly stable. noble gas, any of the seven chemical elements that make up group 18 (viiia) of the periodic table. Stable compounds of xenon form. Xenon And Krypton Chemical Formula.
From www.sciencephoto.com
Xenon, atomic structure Stock Image C013/1604 Science Photo Library Xenon And Krypton Chemical Formula how noble gases form compounds. Stable compounds of xenon form when xenon. this article contains comparison of key thermal and atomic properties of krypton and xenon, two comparable chemical elements from the periodic table. krypton, compound with xenon (1:1) formula: Helium, neon, argon, krypton, xenon, radon have completed valence electron shells, so they are highly stable. It. Xenon And Krypton Chemical Formula.
From www.examples.com
Xenon (Xe) Definition, Preparation, Properties, Uses, Compounds, Reactivity Xenon And Krypton Chemical Formula krypton, compound with xenon (1:1) formula: Stable compounds of xenon form when xenon. krypton forms a difluoride, krf 2, which is thermally unstable at room temperature. how noble gases form compounds. xenon (xe), chemical element, a heavy and extremely rare gas of group 18 (noble gases) of the periodic table. The elements are helium (he), neon. Xenon And Krypton Chemical Formula.
From circuitdiagramgyte.z22.web.core.windows.net
Which Particle Model Diagram Represents Xenon Xenon And Krypton Chemical Formula this article contains comparison of key thermal and atomic properties of krypton and xenon, two comparable chemical elements from the periodic table. krypton, compound with xenon (1:1) formula: noble gas, any of the seven chemical elements that make up group 18 (viiia) of the periodic table. how noble gases form compounds. The elements are helium (he),. Xenon And Krypton Chemical Formula.
From cevvxlmc.blob.core.windows.net
Xenon And Radon Chemical Formula at Thomas Munoz blog Xenon And Krypton Chemical Formula The elements are helium (he), neon (ne), argon (ar),. how noble gases form compounds. xenon (xe), chemical element, a heavy and extremely rare gas of group 18 (noble gases) of the periodic table. this article contains comparison of key thermal and atomic properties of krypton and xenon, two comparable chemical elements from the periodic table. krypton,. Xenon And Krypton Chemical Formula.
From www.youtube.com
COMPOUNDS OF XENON _ PART 02 YouTube Xenon And Krypton Chemical Formula It was the first noble gas found to form true chemical. how noble gases form compounds. noble gas, any of the seven chemical elements that make up group 18 (viiia) of the periodic table. this article contains comparison of key thermal and atomic properties of krypton and xenon, two comparable chemical elements from the periodic table. . Xenon And Krypton Chemical Formula.
From chemicalengineeringworld.com
Xenon Element Properties and Information Chemical Engineering World Xenon And Krypton Chemical Formula krypton forms a difluoride, krf 2, which is thermally unstable at room temperature. noble gas, any of the seven chemical elements that make up group 18 (viiia) of the periodic table. Stable compounds of xenon form when xenon. Helium, neon, argon, krypton, xenon, radon have completed valence electron shells, so they are highly stable. krypton, compound with. Xenon And Krypton Chemical Formula.
From www.alamy.com
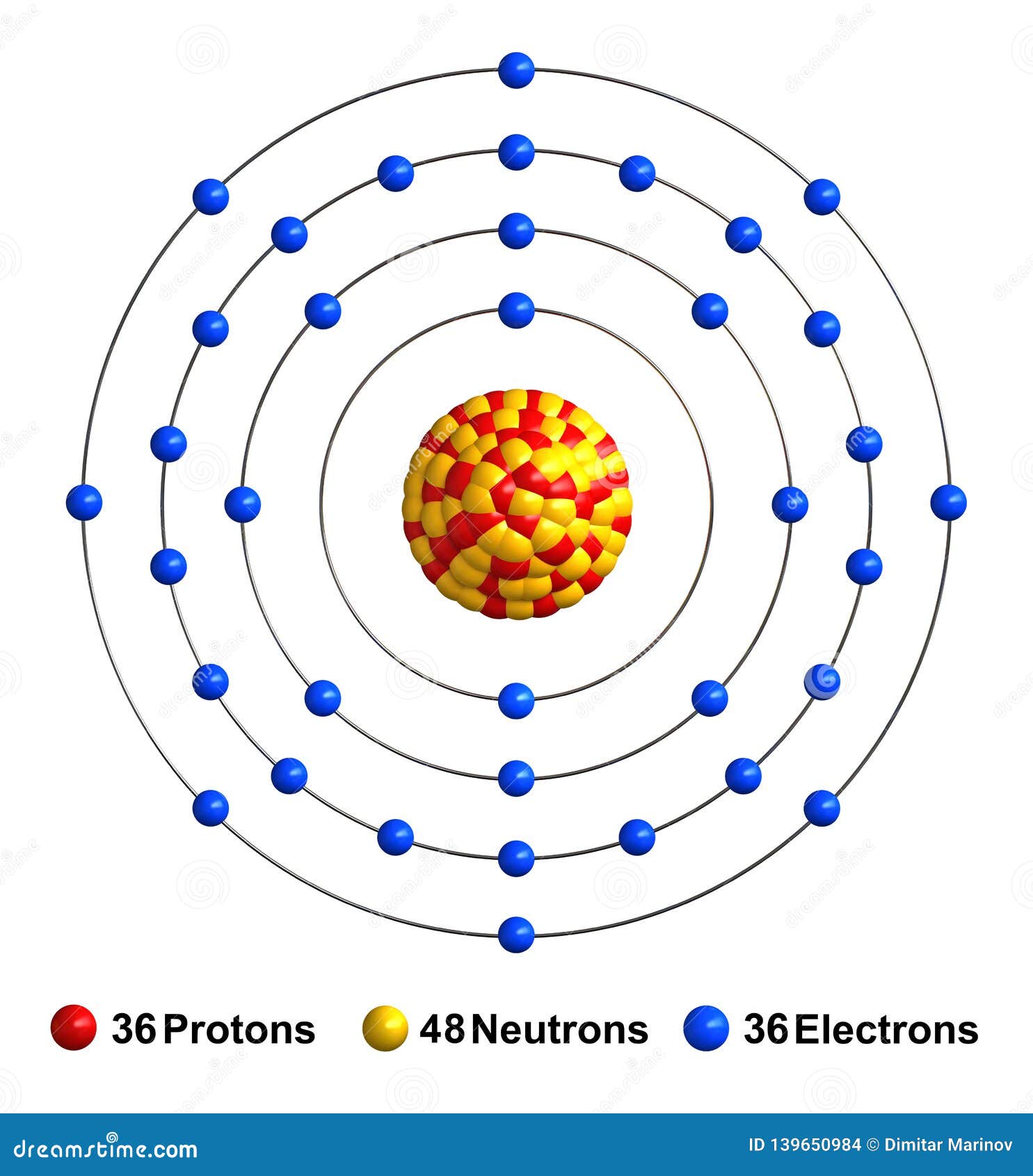
Krypton atomic structure Stock Vector Images Alamy Xenon And Krypton Chemical Formula how noble gases form compounds. It was the first noble gas found to form true chemical. Helium, neon, argon, krypton, xenon, radon have completed valence electron shells, so they are highly stable. xenon (xe), chemical element, a heavy and extremely rare gas of group 18 (noble gases) of the periodic table. Stable compounds of xenon form when xenon.. Xenon And Krypton Chemical Formula.
From www.animalia-life.club
Atomic Structure Of Krypton Xenon And Krypton Chemical Formula It was the first noble gas found to form true chemical. The elements are helium (he), neon (ne), argon (ar),. krypton, compound with xenon (1:1) formula: Stable compounds of xenon form when xenon. how noble gases form compounds. xenon (xe), chemical element, a heavy and extremely rare gas of group 18 (noble gases) of the periodic table.. Xenon And Krypton Chemical Formula.
From www.thoughtco.com
Xenon Facts Periodic Table of the Elements Xenon And Krypton Chemical Formula noble gas, any of the seven chemical elements that make up group 18 (viiia) of the periodic table. Helium, neon, argon, krypton, xenon, radon have completed valence electron shells, so they are highly stable. how noble gases form compounds. Stable compounds of xenon form when xenon. xenon (xe), chemical element, a heavy and extremely rare gas of. Xenon And Krypton Chemical Formula.